[fish meal price](https://www.datawrapper.de/_/TjwV6/)
[fish meal price](https://www.datawrapper.de/_/TjwV6/)
 Tank Specifications:
- Round/oval FRP/fiberglass tanks (2–10 tons capacity).
- Shallow tanks (0.5–1 m depth) for larval rearing.
- Dark-colored tanks (reduces stress in larvae).
---
## 2. Broodstock Collection & Conditioning
### 🔍 Selection Criteria
- Healthy, disease-free adults (no lesions, active movement).
- Mature size:
- *Holothuria scabra*: ≥20 cm, 300–500 g.
- *Holothuria atra*: ≥25 cm, 400–600 g.
### 🌡 Conditioning Process
- Acclimatize in tanks (salinity/temperature matching wild habitat).
- Feed natural diet:
- Seaweed powder (30% protein).
- Microalgae (*Navicula*, *Chaetoceros*).
- Thermal shock method for spawning induction:
- Raise temp to 32°C for 1 hr, then return to 28°C.
---
## 3. Spawning & Fertilization
### ⚡ Induced Spawning Techniques
1. Temperature Shock (Most common)
- Expose to 5°C above ambient for 30–60 mins.
2. Drying + Sunlight Exposure
- Keep broodstock dry for 30 mins, then return to water.
3. Gonad Stimulation (Experimental)
- Serotonin injection (1–2 µg/g body weight).
### 🥚 Egg Collection & Fertilization
- Eggs: 100,000–500,000 per female.
- Sperm: Dilute in seawater before mixing with eggs.
- Fertilization rate: 70–90% under optimal conditions.
---
## 4. Larval Rearing (Auricularia → Pentactula)
| Stage | Duration | Feed | Key Requirements |
|-------|---------|------|-----------------|
| Auricularia | 8–12 days | Microalgae (*Chaetoceros*, *Isochrysis*) | Gentle aeration, 28°C |
| Doliolaria | 3–5 days | Mixed algae + yeast | Reduced light |
| Pentactula | 5–7 days | Benthic diatoms (*Navicula*) | Settlement substrates |
Water Quality:
- pH: 7.8–8.3
- Ammonia: <0.1 ppm
- DO: >5 mg/L
- < 3gr does not need sand Not deposit feeder
- In the wild, juveniles (∼9 mm) settle on seagrass leaves as grazers (Mercier et al., 2000a), and once larger, they migrate onto sand where they become deposit-feeders (Mercier et al., 2000b).
- need different sand size for optimum grow
- can not replace with silica sand
- Sandfish as Deposit Feeders: Sandfish (Holothuria scabra) are a type of sea cucumber that are deposit feeders. This means they eat organic matter, bacteria, microalgae, and other small organisms that live within the sediment (sand) on the seafloor. The sand itself acts as a substrate and a food source, but the nutritional value comes from the living things within it.
- Sargassum as a Fertilizer: Sargassum is a type of seaweed that is rich in nutrients and organic matter. When added to the sandy substrate in sandfish cultivation tanks or ponds, it acts as an organic fertilizer. This enrichment of the sand boosts the growth of the microalgae, bacteria, and other microorganisms that the sandfish feed on. Studies have shown that adding dried sargassum to the sediment can significantly increase the food abundance, leading to faster growth rates for juvenile sandfish.
- Sargassum as a Direct Feed: In addition to fertilizing the sand, sargassum can also be processed into a direct supplemental feed for sandfish. Researchers have tested different preparations, such as fermented sargassum and powdered sargassum, and found that they can improve the growth and survival of sandfish, especially in early juvenile stages. Fermented sargassum, in particular, has been shown to be very effective.
- In summary, sargassum doesn't directly fertilize the sand in the way you might think of a plant fertilizer, but it enriches the organic content of the sand, which in turn acts as a "fertilizer" for the living organisms that the sandfish consume. It can also be a direct and valuable feed source for the sandfish themselves. This use of sargassum is a promising development for sustainable aquaculture and a way to utilize
he large influxes of sargassum that can be a nuisance in coastal areas.
Chlorella vulgaris Powder
- Chlorella vulgaris is a type of microalgae known for its high nutritional value, containing protein, lipids, vitamins, and minerals. While live microalgae are a traditional feed for sandfish larvae and early juveniles in hatcheries, powdered forms like chlorella vulgaris are being explored as a cost-effective alternative.
Usage and Benefits
Supplement to Live Algae: Powdered microalgae can supplement or even replace live microalgae, reducing the high cost and labor associated with maintaining live cultures.
Nutritional Profile: Chlorella is rich in essential amino acids and fatty acids, which are crucial for the growth and health of sandfish. Its high protein content makes it an excellent addition to a sandfish diet.
Improved Growth: Studies on other aquatic species have shown that including Chlorella vulgaris in the diet can enhance growth performance and feed efficiency. This is a promising area for further research in sandfish grow-out.
In summary, both sargassum and chlorella vulgaris powder are valuable feed ingredients for sandfish aquaculture. Sargassum is a well-established and highly effective component, especially when fermented, while chlorella powder is a promising, nutrient-rich supplement that can reduce reliance on expensive live feeds.
---
## 5. Juvenile Settlement & Nursery
### 🪨 Settlement Substrates
- PVC plates (coated with biofilm).
- Coral rubble/seaweed mats (natural texture).
- Sargassum thunbergii & S. polycystum
- Sargassum acts to enrich the sediment is lacking.
## 7. Disease & Biosecurity Management
### 🦠 Common Diseases
- Skin ulceration (bacterial *Vibrio* spp.) → Treat with oxytetracycline baths.
- Parasitic protozoa → Formalin dips (50 ppm for 30 mins).
### 🔒 Biosecurity Measures
- Quarantine new broodstock (7–10 days).
- Disinfect tanks (iodine, hydrogen peroxide).
- PCR testing for pathogens.
---
---
### 3. Transport Conditions
- Temperature: Maintain 26–28°C (use cooling boxes or **insulated containers**).
- Oxygen Monitoring: Ensure O₂ > 5 ppm (use oxygen tablets if needed).
- Avoid Light & Vibration: Keep bags in dark, stable conditions.
---
### 4. Post-Transport Acclimation
#### A. Floating for Temperature Adjustment
- Float unopened bags in destination pond for 15–30 mins to equalize temperature.
#### B. Gradual Salinity Mixing (If Needed)
- Step 1: Open bag, add small amounts of pond water every 5 mins for 30–45 mins.
- Step 2: Release PLs gently near aerated areas.
#### C. Post-Release Care
- PL10: Feed immediately with high-protein diet (e.g., *Artemia*).
- PL15: Can wait 2–3 hrs before feeding (more resilient).
---
### 5. Survival Rate Expectations
| Condition | PL10 Survival (%) | PL15 Survival (%) |
|----------------------|------------------|------------------|
| Ideal Transport | 85–90% | 90–95% |
| Long Haul (>12h) | 70–80% | 85–90% |
---
### Key Differences Summary
| Factor | PL10 Requirements | PL15 Requirements |
|----------------|------------------|------------------|
| Density | Higher (5–10k/L) | Lower (3–5k/L) |
| O₂ Demand | Moderate | Higher |
| Stress | More sensitive | More tolerant |
| Max Time | 6–8 hrs | 12–24 hrs |
Would you like modifications for specific conditions (e.g., air freight, road transport in hot climates)?
LiveSeafood, [6/27/25 10:50 PM]
Here’s a modified transport protocol for specific conditions, including air freight, hot climates, and long-distance road transport, with adjustments for PL10 vs. PL15 *Vannamei* shrimp:
---
### 1. Air Freight Protocol (PL10 & PL15)
#### Key Challenges:
- Pressure changes → Risk of bag rupture.
- Strict airline regulations → Limited oxygen volume.
- No access to temperature control in cargo hold.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Bag Thickness | 0.1–0.15 mm double-layered | 0.15–0.2 mm double-layered |
| Oxygen Ratio | 50% water, 50% O₂ (to prevent bursting) | 40% water, 60% O₂ (more resilient) |
| Density | 3,000–4,000/L (reduce by 30%) | 1,500–2,500/L (reduce by 40%) |
| Additives | 5 ppm EDTA (anti-stress) + 2 ppm probiotics | 3 ppm vitamin C (immune boost) |
| Packaging | Styrofoam box + coolant gel packs (if >6 hrs) | Insulated box + thermal lining |
Note:
- Use oxygen-absorbing pads (for airline safety compliance).
- Avoid ice (risk of cold shock; use chilled gel packs instead).
---
### 2. Hot Climate Transport (Road, >30°C Ambient)
#### Key Challenges:
- Heat stress → Increased metabolism → Oxygen depletion.
- Ammonia spikes due to higher metabolic waste.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Cooling Method | Frozen water bottles (wrapped in cloth) | Phase-change cooling packs |
| Density | Reduce by 40% (e.g., 3,000–4,000/L → 1,800–2,400/L**) | **Reduce by 30% (e.g., **2,000–3,000/L → 1,400–2,100/L**) |
| Oxygen | Pure O₂ + oxygen tablets (as backup) | O₂ with slow-release tablets |
| Additives | 5 ppm sodium bicarbonate (pH buffer) | 2 ppm potassium chloride (ion regulator) |
| Travel Time | Only during cooler hours (night/early morning) | Max 8 hrs (daytime) |
Emergency Measures:
- If bags overheat, gradually lower temperature (avoid sudden cooling).
- Ammonia detox: Add zeolite chips (1–2 g/L) in extreme cases.
---
### 3. Long-Distance Road Transport (>24 Hours)
#### Key Challenges:
- Oxygen depletion → Risk of mass mortality.
- Accumulated waste → Ammonia toxicity.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Density | Max 1,500/L | Max 1,000/L |
| Water Exchange | Not recommended (too stressful) | Partial exchange (10%) after 12 hrs if necessary |
| O₂ Supply | Oxygen generator or cylinder with regulator | O₂ cylinders + diffuser stones |
| Sedation | Avoid (too sensitive) | 1–2 ppm clove oil (if >24 hrs) |
| Monitoring | Check O₂/ammonia every 4 hrs | Check every 6 hrs |
Survival Tips:
- Use battery-powered air pumps for backup aeration.
- Darken transport containers to reduce stress.
---
### 4. Emergency Recovery Protocol
If PLs arrive weak or stressed:
1. Gradual Acclimation:
- Float bags for 45–60 mins (not 30 mins).
- Drip-acclimate with pond water over 1–2 hrs.
2. Revival Additives:
- PL10: 5 ppm glucose + 1 ppm vitamin B12.
- PL15: 3 ppm calcium magnesium + probiotics.
3. Post-Release Care:
- PL10: Hold in nursery tanks (1–2 days) before pond release.
- PL15: Can be released directly but avoid feeding for 4–6 hrs.
---
### Final Recommendations by Condition
| Transport Type | Best for PL10? | Best for PL15? |
|---------------|---------------|---------------|
| Air Freight | Only if <6 hrs | Preferred (more resilient) |
| Hot Climate | Avoid if possible | Use with cooling packs |
| Long Road Haul | Not recommended | Yes (with O₂ support) |
For highest survival:
- PL10: Keep transport <8 hrs, avoid extreme temps.
- PL15: More flexible but monitor O₂ closely.
Would you like a checklist for pre-transport inspection?
Here’s a detailed pre-transport inspection checklist to ensure optimal survival rates for PL10 and PL15 *Vannamei* shrimp during packing and transit:
---
### 🛠 Pre-Transport Inspection Checklist
*(For PL10 & PL15 – Adjust as Needed)*
#### ✅ 1. Shrimp Health Assessment
- PL10:
- ✔️ No visible deformities (e.g., bent rostrum, uneven tails).
- ✔️ Active swimming (≥90% of PLs responsive to light/movement).
- PL15:
✔️ Gills should be clean (no black spots/fouling).
✔️ Full gut line (no empty intestines due to starvation).
#### ✅ 2. Water Quality Verification
- Both PL10 & PL15:
- ✔️ Salinity: Matches source farm (±2 ppt).
- ✔️ Temperature: 26–28°C (no sudden fluctuations).
- ✔️ pH: 7.8–8.2 (use buffering if unstable).
- ✔️ Ammonia/Nitrite: <0.1 ppm (zeolite on standby).
- ✔️ Dissolved O₂: >5 ppm (calibrate meter before packing).
#### ✅ 3. Packing Materials Check
- Bags:
- ✔️ Double-layered polyethylene (≥0.8 mm thickness for road; **1.0 mm for air**).
- ✔️ No leaks (pressure-test with air before filling).
- Oxygen Supply:
- ✔️ Pure O₂ (≥99% purity) + backup oxygen tablets.
- ✔️ Sealing clips/ties (test strength).
- Additives:
- ✔️ Activated charcoal (2–5 g/L) packed separately.
- ✔️ Stress coat (e.g., aloe vera) for PL10.
#### ✅ 4. Environmental Controls
- For Hot Climates:
- ✔️ Frozen gel packs (not ice) wrapped in cloth.
- ✔️ Insulated box with reflector lining (if daytime transport).
- For Air Freight:
- ✔️ Airline-compliant O₂ levels (check IATA regulations).
- ✔️ Pressure-release valve (if high-altitude flight).
- For Long Hauls (>12 hrs):
- ✔️ Battery-powered aerator (fully charged).
- ✔️ Spare O₂ cylinder with regulator.
#### ✅ 5. Logistics & Documentation
- ✔️ Transport permits (if crossing borders).
- ✔️ Emergency contacts (driver/farm/consignee).
- ✔️ Route planned (avoid delays; no direct sunlight exposure).
---
### ⚠️ Red Flags to Cancel Transport
| Issue | PL10 Risk | PL15 Risk |
|-------|----------|----------|
| Ammonia >0.2 ppm | HIGH (mortality ≥50%) | Moderate (mortality 20–30%) |
| Temp >30°C | Extreme stress | Tolerable (if cooled) |
| >10% weak/dead PLs | Do NOT pack | Assess cause first |
---
### 📌 Post-Checklist Actions
1. PL10-Specific:
- Conduct 1-hour test pack with 1 bag (monitor O₂ drop rate).
- Feed high-protein diet 12 hrs pre-transport (then starve).
2. PL15-Specific:
- Check for exuviae (molts) in holding tank (indicates health).
- Sedate only if transport >24 hrs (**1 ppm clove oil**).
---
### 🔧 Emergency Kit for Transport Team
- For PL10:
- Portable O₂ meter + ammonia test strips.
- Glucose solution (for revival).
- For PL15:
- Spare battery-powered air pump.
- Calcium magnesium (for osmotic balance).
---
Final Tip: For PL10, prioritize shorter, cooler, and stable transports. PL15 can handle tougher conditions but still require monitoring.
Would you like a printable PDF version of this checklist?
LiveSeafood, [6/27/25 10:53 PM]
Here’s a printable PDF-friendly version of the checklist. You can copy this into a document, adjust formatting, and save as a PDF:
---
# 🦐 Pre-Transport Checklist for Vannamei PL10 & PL15
*(Print Before Each Shipment)*
### 📅 Date: _____
### 🔢 Batch No.: _____
### 🛣 Transport Type: ☐ Air ☐ Road ☐ Hot Climate ☐ Long Haul (>12h)
---
---
https://www.globalseafood.org/advocate/trials-test-effects-of-hormone-supplemented-feed-on-sex-reversal-of-freshwater-prawns/

[brine shrimp sieve](https://www.brineshrimpdirect.com/rotifer-sieve)
[green freshwater algae](https://www.e3s-conferences.org/articles/e3sconf/pdf/2018/06/e3sconf_icenis2018_04009.pdf)
Tank Specifications:
- Round/oval FRP/fiberglass tanks (2–10 tons capacity).
- Shallow tanks (0.5–1 m depth) for larval rearing.
- Dark-colored tanks (reduces stress in larvae).
---
## 2. Broodstock Collection & Conditioning
### 🔍 Selection Criteria
- Healthy, disease-free adults (no lesions, active movement).
- Mature size:
- *Holothuria scabra*: ≥20 cm, 300–500 g.
- *Holothuria atra*: ≥25 cm, 400–600 g.
### 🌡 Conditioning Process
- Acclimatize in tanks (salinity/temperature matching wild habitat).
- Feed natural diet:
- Seaweed powder (30% protein).
- Microalgae (*Navicula*, *Chaetoceros*).
- Thermal shock method for spawning induction:
- Raise temp to 32°C for 1 hr, then return to 28°C.
---
## 3. Spawning & Fertilization
### ⚡ Induced Spawning Techniques
1. Temperature Shock (Most common)
- Expose to 5°C above ambient for 30–60 mins.
2. Drying + Sunlight Exposure
- Keep broodstock dry for 30 mins, then return to water.
3. Gonad Stimulation (Experimental)
- Serotonin injection (1–2 µg/g body weight).
### 🥚 Egg Collection & Fertilization
- Eggs: 100,000–500,000 per female.
- Sperm: Dilute in seawater before mixing with eggs.
- Fertilization rate: 70–90% under optimal conditions.
---
## 4. Larval Rearing (Auricularia → Pentactula)
| Stage | Duration | Feed | Key Requirements |
|-------|---------|------|-----------------|
| Auricularia | 8–12 days | Microalgae (*Chaetoceros*, *Isochrysis*) | Gentle aeration, 28°C |
| Doliolaria | 3–5 days | Mixed algae + yeast | Reduced light |
| Pentactula | 5–7 days | Benthic diatoms (*Navicula*) | Settlement substrates |
Water Quality:
- pH: 7.8–8.3
- Ammonia: <0.1 ppm
- DO: >5 mg/L
- < 3gr does not need sand Not deposit feeder
- In the wild, juveniles (∼9 mm) settle on seagrass leaves as grazers (Mercier et al., 2000a), and once larger, they migrate onto sand where they become deposit-feeders (Mercier et al., 2000b).
- need different sand size for optimum grow
- can not replace with silica sand
- Sandfish as Deposit Feeders: Sandfish (Holothuria scabra) are a type of sea cucumber that are deposit feeders. This means they eat organic matter, bacteria, microalgae, and other small organisms that live within the sediment (sand) on the seafloor. The sand itself acts as a substrate and a food source, but the nutritional value comes from the living things within it.
- Sargassum as a Fertilizer: Sargassum is a type of seaweed that is rich in nutrients and organic matter. When added to the sandy substrate in sandfish cultivation tanks or ponds, it acts as an organic fertilizer. This enrichment of the sand boosts the growth of the microalgae, bacteria, and other microorganisms that the sandfish feed on. Studies have shown that adding dried sargassum to the sediment can significantly increase the food abundance, leading to faster growth rates for juvenile sandfish.
- Sargassum as a Direct Feed: In addition to fertilizing the sand, sargassum can also be processed into a direct supplemental feed for sandfish. Researchers have tested different preparations, such as fermented sargassum and powdered sargassum, and found that they can improve the growth and survival of sandfish, especially in early juvenile stages. Fermented sargassum, in particular, has been shown to be very effective.
- In summary, sargassum doesn't directly fertilize the sand in the way you might think of a plant fertilizer, but it enriches the organic content of the sand, which in turn acts as a "fertilizer" for the living organisms that the sandfish consume. It can also be a direct and valuable feed source for the sandfish themselves. This use of sargassum is a promising development for sustainable aquaculture and a way to utilize
he large influxes of sargassum that can be a nuisance in coastal areas.
Chlorella vulgaris Powder
- Chlorella vulgaris is a type of microalgae known for its high nutritional value, containing protein, lipids, vitamins, and minerals. While live microalgae are a traditional feed for sandfish larvae and early juveniles in hatcheries, powdered forms like chlorella vulgaris are being explored as a cost-effective alternative.
Usage and Benefits
Supplement to Live Algae: Powdered microalgae can supplement or even replace live microalgae, reducing the high cost and labor associated with maintaining live cultures.
Nutritional Profile: Chlorella is rich in essential amino acids and fatty acids, which are crucial for the growth and health of sandfish. Its high protein content makes it an excellent addition to a sandfish diet.
Improved Growth: Studies on other aquatic species have shown that including Chlorella vulgaris in the diet can enhance growth performance and feed efficiency. This is a promising area for further research in sandfish grow-out.
In summary, both sargassum and chlorella vulgaris powder are valuable feed ingredients for sandfish aquaculture. Sargassum is a well-established and highly effective component, especially when fermented, while chlorella powder is a promising, nutrient-rich supplement that can reduce reliance on expensive live feeds.
---
## 5. Juvenile Settlement & Nursery
### 🪨 Settlement Substrates
- PVC plates (coated with biofilm).
- Coral rubble/seaweed mats (natural texture).
- Sargassum thunbergii & S. polycystum
- Sargassum acts to enrich the sediment is lacking.
## 7. Disease & Biosecurity Management
### 🦠 Common Diseases
- Skin ulceration (bacterial *Vibrio* spp.) → Treat with oxytetracycline baths.
- Parasitic protozoa → Formalin dips (50 ppm for 30 mins).
### 🔒 Biosecurity Measures
- Quarantine new broodstock (7–10 days).
- Disinfect tanks (iodine, hydrogen peroxide).
- PCR testing for pathogens.
---
---
### 3. Transport Conditions
- Temperature: Maintain 26–28°C (use cooling boxes or **insulated containers**).
- Oxygen Monitoring: Ensure O₂ > 5 ppm (use oxygen tablets if needed).
- Avoid Light & Vibration: Keep bags in dark, stable conditions.
---
### 4. Post-Transport Acclimation
#### A. Floating for Temperature Adjustment
- Float unopened bags in destination pond for 15–30 mins to equalize temperature.
#### B. Gradual Salinity Mixing (If Needed)
- Step 1: Open bag, add small amounts of pond water every 5 mins for 30–45 mins.
- Step 2: Release PLs gently near aerated areas.
#### C. Post-Release Care
- PL10: Feed immediately with high-protein diet (e.g., *Artemia*).
- PL15: Can wait 2–3 hrs before feeding (more resilient).
---
### 5. Survival Rate Expectations
| Condition | PL10 Survival (%) | PL15 Survival (%) |
|----------------------|------------------|------------------|
| Ideal Transport | 85–90% | 90–95% |
| Long Haul (>12h) | 70–80% | 85–90% |
---
### Key Differences Summary
| Factor | PL10 Requirements | PL15 Requirements |
|----------------|------------------|------------------|
| Density | Higher (5–10k/L) | Lower (3–5k/L) |
| O₂ Demand | Moderate | Higher |
| Stress | More sensitive | More tolerant |
| Max Time | 6–8 hrs | 12–24 hrs |
Would you like modifications for specific conditions (e.g., air freight, road transport in hot climates)?
LiveSeafood, [6/27/25 10:50 PM]
Here’s a modified transport protocol for specific conditions, including air freight, hot climates, and long-distance road transport, with adjustments for PL10 vs. PL15 *Vannamei* shrimp:
---
### 1. Air Freight Protocol (PL10 & PL15)
#### Key Challenges:
- Pressure changes → Risk of bag rupture.
- Strict airline regulations → Limited oxygen volume.
- No access to temperature control in cargo hold.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Bag Thickness | 0.1–0.15 mm double-layered | 0.15–0.2 mm double-layered |
| Oxygen Ratio | 50% water, 50% O₂ (to prevent bursting) | 40% water, 60% O₂ (more resilient) |
| Density | 3,000–4,000/L (reduce by 30%) | 1,500–2,500/L (reduce by 40%) |
| Additives | 5 ppm EDTA (anti-stress) + 2 ppm probiotics | 3 ppm vitamin C (immune boost) |
| Packaging | Styrofoam box + coolant gel packs (if >6 hrs) | Insulated box + thermal lining |
Note:
- Use oxygen-absorbing pads (for airline safety compliance).
- Avoid ice (risk of cold shock; use chilled gel packs instead).
---
### 2. Hot Climate Transport (Road, >30°C Ambient)
#### Key Challenges:
- Heat stress → Increased metabolism → Oxygen depletion.
- Ammonia spikes due to higher metabolic waste.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Cooling Method | Frozen water bottles (wrapped in cloth) | Phase-change cooling packs |
| Density | Reduce by 40% (e.g., 3,000–4,000/L → 1,800–2,400/L**) | **Reduce by 30% (e.g., **2,000–3,000/L → 1,400–2,100/L**) |
| Oxygen | Pure O₂ + oxygen tablets (as backup) | O₂ with slow-release tablets |
| Additives | 5 ppm sodium bicarbonate (pH buffer) | 2 ppm potassium chloride (ion regulator) |
| Travel Time | Only during cooler hours (night/early morning) | Max 8 hrs (daytime) |
Emergency Measures:
- If bags overheat, gradually lower temperature (avoid sudden cooling).
- Ammonia detox: Add zeolite chips (1–2 g/L) in extreme cases.
---
### 3. Long-Distance Road Transport (>24 Hours)
#### Key Challenges:
- Oxygen depletion → Risk of mass mortality.
- Accumulated waste → Ammonia toxicity.
#### Adjustments:
| Factor | PL10 | PL15 |
|--------|------|------|
| Density | Max 1,500/L | Max 1,000/L |
| Water Exchange | Not recommended (too stressful) | Partial exchange (10%) after 12 hrs if necessary |
| O₂ Supply | Oxygen generator or cylinder with regulator | O₂ cylinders + diffuser stones |
| Sedation | Avoid (too sensitive) | 1–2 ppm clove oil (if >24 hrs) |
| Monitoring | Check O₂/ammonia every 4 hrs | Check every 6 hrs |
Survival Tips:
- Use battery-powered air pumps for backup aeration.
- Darken transport containers to reduce stress.
---
### 4. Emergency Recovery Protocol
If PLs arrive weak or stressed:
1. Gradual Acclimation:
- Float bags for 45–60 mins (not 30 mins).
- Drip-acclimate with pond water over 1–2 hrs.
2. Revival Additives:
- PL10: 5 ppm glucose + 1 ppm vitamin B12.
- PL15: 3 ppm calcium magnesium + probiotics.
3. Post-Release Care:
- PL10: Hold in nursery tanks (1–2 days) before pond release.
- PL15: Can be released directly but avoid feeding for 4–6 hrs.
---
### Final Recommendations by Condition
| Transport Type | Best for PL10? | Best for PL15? |
|---------------|---------------|---------------|
| Air Freight | Only if <6 hrs | Preferred (more resilient) |
| Hot Climate | Avoid if possible | Use with cooling packs |
| Long Road Haul | Not recommended | Yes (with O₂ support) |
For highest survival:
- PL10: Keep transport <8 hrs, avoid extreme temps.
- PL15: More flexible but monitor O₂ closely.
Would you like a checklist for pre-transport inspection?
Here’s a detailed pre-transport inspection checklist to ensure optimal survival rates for PL10 and PL15 *Vannamei* shrimp during packing and transit:
---
### 🛠 Pre-Transport Inspection Checklist
*(For PL10 & PL15 – Adjust as Needed)*
#### ✅ 1. Shrimp Health Assessment
- PL10:
- ✔️ No visible deformities (e.g., bent rostrum, uneven tails).
- ✔️ Active swimming (≥90% of PLs responsive to light/movement).
- PL15:
✔️ Gills should be clean (no black spots/fouling).
✔️ Full gut line (no empty intestines due to starvation).
#### ✅ 2. Water Quality Verification
- Both PL10 & PL15:
- ✔️ Salinity: Matches source farm (±2 ppt).
- ✔️ Temperature: 26–28°C (no sudden fluctuations).
- ✔️ pH: 7.8–8.2 (use buffering if unstable).
- ✔️ Ammonia/Nitrite: <0.1 ppm (zeolite on standby).
- ✔️ Dissolved O₂: >5 ppm (calibrate meter before packing).
#### ✅ 3. Packing Materials Check
- Bags:
- ✔️ Double-layered polyethylene (≥0.8 mm thickness for road; **1.0 mm for air**).
- ✔️ No leaks (pressure-test with air before filling).
- Oxygen Supply:
- ✔️ Pure O₂ (≥99% purity) + backup oxygen tablets.
- ✔️ Sealing clips/ties (test strength).
- Additives:
- ✔️ Activated charcoal (2–5 g/L) packed separately.
- ✔️ Stress coat (e.g., aloe vera) for PL10.
#### ✅ 4. Environmental Controls
- For Hot Climates:
- ✔️ Frozen gel packs (not ice) wrapped in cloth.
- ✔️ Insulated box with reflector lining (if daytime transport).
- For Air Freight:
- ✔️ Airline-compliant O₂ levels (check IATA regulations).
- ✔️ Pressure-release valve (if high-altitude flight).
- For Long Hauls (>12 hrs):
- ✔️ Battery-powered aerator (fully charged).
- ✔️ Spare O₂ cylinder with regulator.
#### ✅ 5. Logistics & Documentation
- ✔️ Transport permits (if crossing borders).
- ✔️ Emergency contacts (driver/farm/consignee).
- ✔️ Route planned (avoid delays; no direct sunlight exposure).
---
### ⚠️ Red Flags to Cancel Transport
| Issue | PL10 Risk | PL15 Risk |
|-------|----------|----------|
| Ammonia >0.2 ppm | HIGH (mortality ≥50%) | Moderate (mortality 20–30%) |
| Temp >30°C | Extreme stress | Tolerable (if cooled) |
| >10% weak/dead PLs | Do NOT pack | Assess cause first |
---
### 📌 Post-Checklist Actions
1. PL10-Specific:
- Conduct 1-hour test pack with 1 bag (monitor O₂ drop rate).
- Feed high-protein diet 12 hrs pre-transport (then starve).
2. PL15-Specific:
- Check for exuviae (molts) in holding tank (indicates health).
- Sedate only if transport >24 hrs (**1 ppm clove oil**).
---
### 🔧 Emergency Kit for Transport Team
- For PL10:
- Portable O₂ meter + ammonia test strips.
- Glucose solution (for revival).
- For PL15:
- Spare battery-powered air pump.
- Calcium magnesium (for osmotic balance).
---
Final Tip: For PL10, prioritize shorter, cooler, and stable transports. PL15 can handle tougher conditions but still require monitoring.
Would you like a printable PDF version of this checklist?
LiveSeafood, [6/27/25 10:53 PM]
Here’s a printable PDF-friendly version of the checklist. You can copy this into a document, adjust formatting, and save as a PDF:
---
# 🦐 Pre-Transport Checklist for Vannamei PL10 & PL15
*(Print Before Each Shipment)*
### 📅 Date: _____
### 🔢 Batch No.: _____
### 🛣 Transport Type: ☐ Air ☐ Road ☐ Hot Climate ☐ Long Haul (>12h)
---
---
https://www.globalseafood.org/advocate/trials-test-effects-of-hormone-supplemented-feed-on-sex-reversal-of-freshwater-prawns/

[brine shrimp sieve](https://www.brineshrimpdirect.com/rotifer-sieve)
[green freshwater algae](https://www.e3s-conferences.org/articles/e3sconf/pdf/2018/06/e3sconf_icenis2018_04009.pdf)
graph LR;
%% Ocean sea water
%% x
subgraph Pacific["Pacific-Indonesia"]
direction LR
ocean_Temperature["Pacific surface T= approx 26~30C"]
end
click ocean_Temperature "https://www.globalseafood.org/advocate/trials-test-effects-of-hormone-supplemented-feed-on-sex-reversal-of-freshwater-prawns/" "Surface ocean temperature"
---
graph LR;
%% Freshwater prawn sex reversal
%% x
subgraph SexReverse["Sex Reversal"]
direction LR
prawn_Male["Methyltestosterone experiment"]
prawn_Female["Dopamine experiment
Ovaprim Fish Hormone Domperidone Sgnrha Injection"] sex_reverse_for_tilapia["Tilapia sex reversal in fry stage"] end FWprawn["Mixed Culture"] FWprawn_M["Male mono culture
Teritorial"] FWprawn_F["Female mono culture
higher density
more uniform
less teritorial vs male"] click SexReverse "https://www.globalseafood.org/advocate/trials-test-effects-of-hormone-supplemented-feed-on-sex-reversal-of-freshwater-prawns/" "SexReversal Post Larva" click sex_reverse_for_tilapia "https://www.globalseafood.org/advocate/artificial-incubation-hormonal-sex-reversal-promoted-tilapia-boom/" "Sex reversal for tilapia"
Ovaprim Fish Hormone Domperidone Sgnrha Injection"] sex_reverse_for_tilapia["Tilapia sex reversal in fry stage"] end FWprawn["Mixed Culture"] FWprawn_M["Male mono culture
Teritorial"] FWprawn_F["Female mono culture
higher density
more uniform
less teritorial vs male"] click SexReverse "https://www.globalseafood.org/advocate/trials-test-effects-of-hormone-supplemented-feed-on-sex-reversal-of-freshwater-prawns/" "SexReversal Post Larva" click sex_reverse_for_tilapia "https://www.globalseafood.org/advocate/artificial-incubation-hormonal-sex-reversal-promoted-tilapia-boom/" "Sex reversal for tilapia"
graph LR
%% Benur vannamei
BenurVPL_10["Vannamei Post Larva -10 days"]
BenurVPL_15["Vannamei Post Larva - 15 days"]
BenurVPL_10 ---> BenurVPL_15
BenurVPL_10 --->|"sell and ship to customer"| qnt_1_1
BenurVPL_15 --->|"sell and ship to customer"| qnt_1_2
subgraph select_req1_steps ["selection criteria"]
direction LR
select_strong["discard slow molting PL"]
end
BenurVPL_10 <---> select_req1_steps
subgraph shipment_req1_steps ["shipment to customer PL10 < 6 ~ 8 hours
PL-15 < 12~24 hours"] direction LR Stress_Test_1_1["stress test prior to packing
salinity test
salinity 0, 30 min then tank salinity 30 min
count life > 85%"] weight_Test_1_1["number of PL count per PL1,Pl10,P12,P? statistics"] salinity["Both require 26–30°C and salinity 25–35 ppt"] qnt_1_1["PL-10 qnty: 5000-10000/L(seawater-1 L)
+2/3 filled with O2(2L)"] qnt_1_2["PL-15 qnty: 3000-5000/L(seawater-1 L)
2/3 filled with O2(2L)"] end click BenurVPL_10 "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Vannemei Benur PL-10" click BenurVPL_15 "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Vannemei Benur PL-15"
---
PL-15 < 12~24 hours"] direction LR Stress_Test_1_1["stress test prior to packing
salinity test
salinity 0, 30 min then tank salinity 30 min
count life > 85%"] weight_Test_1_1["number of PL count per PL1,Pl10,P12,P? statistics"] salinity["Both require 26–30°C and salinity 25–35 ppt"] qnt_1_1["PL-10 qnty: 5000-10000/L(seawater-1 L)
+2/3 filled with O2(2L)"] qnt_1_2["PL-15 qnty: 3000-5000/L(seawater-1 L)
2/3 filled with O2(2L)"] end click BenurVPL_10 "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Vannemei Benur PL-10" click BenurVPL_15 "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Vannemei Benur PL-15"
graph LR
%% Kerapu and seabass
%% seacucumber deposit feeder. Its diet consists of organic matter and microorganisms that live on and in the sand (the substrate).
FC0["Floating cage
Grading"] FC1["Keramba tancap
Berpasir dibawah
Grading"] FC2["Keramba dasar (0.5 m dari dasar)
Grading"] P0["Pond - outdoor"] P1(["Pond - indoor"]) subgraph pythoplankton["Marine Phytoplankton and/or freshwater pythoplanton
Media for MicroAlgae"] direction LR %% Microalgae cultivation can be carried out with freshwater, seawater, water from estuaries, wastewater from agricultural, industrial operations and domestic wastewater [15]. %% Algae media refers to the solution or culture in which algae grow. %% Nutrient that contained in the tofu wastewater are very useful for the production of Chlorella sp. %% Chlorella sp. usually grows in seawater culture_both["Chlorella vulgaris
is green freshwater microalgae
but seawater tolerance T: 26-30C
an remove salt"] culture_pytho["Culture Marine pythoplankton/MicroAlgae (chlorella sp)"] culture_spiriluna["Spirulina platensis microalgee Blue-green
brackish tolerance
pH sensitivity pH:8~11,T:30-35C"] %% Spiriluna need to adjust fertelizer with pH above seawater %% EC: 98~30ms/cm %% High temperature: 30-35C culture_fresh_pytho["culture freshwater microalgae Spirulina Plantis and Chlorella vulgaris"] end subgraph MacroAlgae["culture seaweeds/MacroAlage"] direction LR seaweed["life green/brown seaweed"] -->Powder_seaweed["Make dry powder seaweed"] seaweed---> Process_1["harvest the seed for future propagation"] end subgraph plankton["Zooplankton Culture"] R0{"Rotifer Large size 20-25°C
Euryhaline"} R1{"Rotifer Small size 28-35°C
Low salinity"} Copepods{"Copepods Bigger than Rotifer"} MicroAlgae["Green phytoplanktons"] --->R0 MicroAlgae -->R1 MicroAlgae ----> Copepods end Seabass1[/"Seabass Mature change sex 5kg ♀
Broodstock Rearing"/] Kerapu1[/"Grouper Mature change sex ♂
Broodstock Rearing"/] Kerapu(["Grouper: female at birth: ♀
Larva Rearing"]) --> |"size: 8-12cm cuci air tawar"| FC0 --> |"mature/adult size"| FC22["floating cage bigger net size
and/or tambak lebih besar ↑O2
Brookstock around 3kg"] Kerapu -->|tidak perlu| FC1 --> FC22 --> Kerapu1 Seabass(["Asian Seabass: Male at birth: ♂
Larva Rearing"]) --> |"size: 8 - 12cm bersisik tidak perlu pasir"| FC0 -->|"Mature/adult size"| FC22 --> Seabass1 Lobster["Lobster Rearing"] -->FC2 click culture_fresh_pytho "https://algaeresearchsupply.com/products/algae-research-supply-algae-culture-chlorella-pyrenoidosa?srsltid=AfmBOoqYbKG86RMM1kP_KYZvTHlMmcgQWKry5cNg-7a28B-AxddtVZfK" "culture Chlorella" click Seabass "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Seabass Hatchery" click Kerapu "https://www.seafdec.org.ph/hatchery-seedstock/" "Grouper Hatchery" style Kerapu1 fill:gold, color:black style Seabass1 fill:gold, color:black style R0 fill:blue, color:white style R1 fill:green, color: black style culture_both fill:green, color:white
Grading"] FC1["Keramba tancap
Berpasir dibawah
Grading"] FC2["Keramba dasar (0.5 m dari dasar)
Grading"] P0["Pond - outdoor"] P1(["Pond - indoor"]) subgraph pythoplankton["Marine Phytoplankton and/or freshwater pythoplanton
Media for MicroAlgae"] direction LR %% Microalgae cultivation can be carried out with freshwater, seawater, water from estuaries, wastewater from agricultural, industrial operations and domestic wastewater [15]. %% Algae media refers to the solution or culture in which algae grow. %% Nutrient that contained in the tofu wastewater are very useful for the production of Chlorella sp. %% Chlorella sp. usually grows in seawater culture_both["Chlorella vulgaris
is green freshwater microalgae
but seawater tolerance T: 26-30C
an remove salt"] culture_pytho["Culture Marine pythoplankton/MicroAlgae (chlorella sp)"] culture_spiriluna["Spirulina platensis microalgee Blue-green
brackish tolerance
pH sensitivity pH:8~11,T:30-35C"] %% Spiriluna need to adjust fertelizer with pH above seawater %% EC: 98~30ms/cm %% High temperature: 30-35C culture_fresh_pytho["culture freshwater microalgae Spirulina Plantis and Chlorella vulgaris"] end subgraph MacroAlgae["culture seaweeds/MacroAlage"] direction LR seaweed["life green/brown seaweed"] -->Powder_seaweed["Make dry powder seaweed"] seaweed---> Process_1["harvest the seed for future propagation"] end subgraph plankton["Zooplankton Culture"] R0{"Rotifer Large size 20-25°C
Euryhaline"} R1{"Rotifer Small size 28-35°C
Low salinity"} Copepods{"Copepods Bigger than Rotifer"} MicroAlgae["Green phytoplanktons"] --->R0 MicroAlgae -->R1 MicroAlgae ----> Copepods end Seabass1[/"Seabass Mature change sex 5kg ♀
Broodstock Rearing"/] Kerapu1[/"Grouper Mature change sex ♂
Broodstock Rearing"/] Kerapu(["Grouper: female at birth: ♀
Larva Rearing"]) --> |"size: 8-12cm cuci air tawar"| FC0 --> |"mature/adult size"| FC22["floating cage bigger net size
and/or tambak lebih besar ↑O2
Brookstock around 3kg"] Kerapu -->|tidak perlu| FC1 --> FC22 --> Kerapu1 Seabass(["Asian Seabass: Male at birth: ♂
Larva Rearing"]) --> |"size: 8 - 12cm bersisik tidak perlu pasir"| FC0 -->|"Mature/adult size"| FC22 --> Seabass1 Lobster["Lobster Rearing"] -->FC2 click culture_fresh_pytho "https://algaeresearchsupply.com/products/algae-research-supply-algae-culture-chlorella-pyrenoidosa?srsltid=AfmBOoqYbKG86RMM1kP_KYZvTHlMmcgQWKry5cNg-7a28B-AxddtVZfK" "culture Chlorella" click Seabass "https://www.seafdec.org.ph/2011/seabass-hatchery/" "Seabass Hatchery" click Kerapu "https://www.seafdec.org.ph/hatchery-seedstock/" "Grouper Hatchery" style Kerapu1 fill:gold, color:black style Seabass1 fill:gold, color:black style R0 fill:blue, color:white style R1 fill:green, color: black style culture_both fill:green, color:white
flowchart TD
T["Tilapia
iBEST (all Male) or
iEXCEL (Mixed-sex)"] subgraph plankton["plankton feed to larvae"] PyPL1["Pythoplanton"] R0{"Rotifer Large ΔT,
20°C-25°C,Euryhaline
B. plicatilis (L)
171-238µm "} R1{"Rotifer Small ΔT,
28°C-35°C, low salinity
B. rotundiformis (S)
121-162µm"} Copepods["Copepods"] Artemia["Artemia
Brine shrmp"] eggs["Fish Eggs"] PyPL1 --> Artemia PyPL1 --> R0 PyPL1 --> R1 end subgraph hatchery["Hatchery Market Depend on Market"] direction LR Eggs["Eggs"] --> Larvae["Post Larvae"] -->Juvinille["Juvinille"] end subgraph growup ["growup Market size"] direction LR Market["different stage of Market"] end subgraph artemiaGrowth ["Artemeia Growth up Culture"] direction LR artemeia_eggs["Artemaia Eggs and larvae for feed"] end subgraph vitamin ["Vitamin C and B12 complex"] direction LR vitamin_feed["feed to rotifer prior to the feed for Hatchery"] end subgraph Pellet_Custard ["Pellet or Custaard"] direction LR Pellet_feed["Pellet feed"] custard["wet or frozen feed"] end T --> hatchery plankton --> hatchery hatchery --> growup style eggs fill:pink, color:black click hatchery "https://www.seafdec.org.ph/hatchery-seedstock/" "Grouper Hatchery" click Copepods "https://animalfact.com/wp-content/uploads/2024/06/Copepods-Copepoda.jpg" "Copepods"
iBEST (all Male) or
iEXCEL (Mixed-sex)"] subgraph plankton["plankton feed to larvae"] PyPL1["Pythoplanton"] R0{"Rotifer Large ΔT,
20°C-25°C,Euryhaline
B. plicatilis (L)
171-238µm "} R1{"Rotifer Small ΔT,
28°C-35°C, low salinity
B. rotundiformis (S)
121-162µm"} Copepods["Copepods"] Artemia["Artemia
Brine shrmp"] eggs["Fish Eggs"] PyPL1 --> Artemia PyPL1 --> R0 PyPL1 --> R1 end subgraph hatchery["Hatchery Market Depend on Market"] direction LR Eggs["Eggs"] --> Larvae["Post Larvae"] -->Juvinille["Juvinille"] end subgraph growup ["growup Market size"] direction LR Market["different stage of Market"] end subgraph artemiaGrowth ["Artemeia Growth up Culture"] direction LR artemeia_eggs["Artemaia Eggs and larvae for feed"] end subgraph vitamin ["Vitamin C and B12 complex"] direction LR vitamin_feed["feed to rotifer prior to the feed for Hatchery"] end subgraph Pellet_Custard ["Pellet or Custaard"] direction LR Pellet_feed["Pellet feed"] custard["wet or frozen feed"] end T --> hatchery plankton --> hatchery hatchery --> growup style eggs fill:pink, color:black click hatchery "https://www.seafdec.org.ph/hatchery-seedstock/" "Grouper Hatchery" click Copepods "https://animalfact.com/wp-content/uploads/2024/06/Copepods-Copepoda.jpg" "Copepods"
flowchart TB
subgraph OCEAN9 ["fas:fa-ship OCEAN 9 "]
direction LR
TP([Telaga Punggur])-->|ferry 11:00 web kapaltiket.com| A([BENAN]) --> |"00:45"| B([Tajuh Biru]) --> C(["Sei Tenam"]) --> D(["Senayang"]) <--->|"from 07:15 Pancur"| E(["Pancur"])
end
subgraph DRAGON5 ["fas:fa-ship DRAGON 5"]
direction LR
TP1(["Telaga punggur"]) --> CC(["Sei Tenam"])-->JG(["Jagoh"])
end
subgraph Anambas ["fas:fa-ship Anambas"]
direction LR
TP2(["Telaga Punggur"]) --"Rp. 494k"--> Letung --"Rp 495k"--> Terempa(["Terempa"])
end
subgraph Bintan["Pulau Bintan/Tanjung Pinang"]
direction LR
BTP(["Tanjung Pinang"])
end
TP <--.--> TP1 <--.--> TP2
style A fill: black, color: white
style B fill:grey, color:#fff
style TP fill:blue, color: whit
style TP1 fill:blue, color: white
style TP2 fill:blue, color: white
click BTP "https://www.welcometobintan.com/images/map.jpg" "Tanjung Pinang"
deposit feeder. Its diet consists of organic matter and microorganisms that live on and in the sand (the substrate).
flowchart LR
subgraph subgraph1["Benur Bening Lobster"]
direction TB
top1["🦞 ≤ 2 gr (≤ 2months)
SR 30%~40%
Nature < 0.01%"] --> bottom1["🦞 ≥ 5gr (2 - 3 months) SR ≥ 70%~80%"] end subgraph subgraph2["Top floating or/and
Bottom floating cage(Krankeng)"] direction TB top2[top] --> bottom2[bottom] end subgraph subgraph3["Top floating or/and
Bottom floating cage(Krankeng)"] direction TB top3[topx] --> bottom3[bottomx] end outside:::asNode --> subgraph1:::asNode outside ---> top2 -->top3 style subgraph2 fill:orange classDef asNode fill:grey, color:orange
SR 30%~40%
Nature < 0.01%"] --> bottom1["🦞 ≥ 5gr (2 - 3 months) SR ≥ 70%~80%"] end subgraph subgraph2["Top floating or/and
Bottom floating cage(Krankeng)"] direction TB top2[top] --> bottom2[bottom] end subgraph subgraph3["Top floating or/and
Bottom floating cage(Krankeng)"] direction TB top3[topx] --> bottom3[bottomx] end outside:::asNode --> subgraph1:::asNode outside ---> top2 -->top3 style subgraph2 fill:orange classDef asNode fill:grey, color:orange
flowchart LR
deposit_feeder("Deposit Feeder
seacucumber")
seacucumber")
pie title Investment ssharing
"PT Innovare" :75
"Mitra" : 15
"Desa" : 10
 |
|  |
- some text
| Column One | Column Two |
| --- | --- |
| data cell one | data cell two |
③ ④ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩
⓪
① ② ③ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩
⑪ ⑫ ⑬ ⑭ ⑮ ⑯ ⑰ ⑱ ⑲
🄰 🄱 🄲 🄳 🄴 🄵 🄶 🄷 🄸 🄹 🄺 🄻 🄼 🄽 🄾 🄿 🅀 🅁 🅂 🅃 🅄 🅅 🅆 🅇 🅈 🅉
Ⓐ Ⓑ Ⓒ Ⓓ Ⓔ
⓿
❶ ❷ ❸ ❹ ❺ ❻ ❼ ❽ ❾ ❿
⓫ ⓬ ⓭ ⓮ ⓯ ⓰ ⓱ ⓲ ⓳ ⓴
Think of Pieces as a technical Tinkerbell from Peter Pan[^2].
[^2]: This is a fairytale about a forever young boy who takes a group of British children to his home island, Neverland.
Depending on how much cookie dough you eat, you’ll have about ==20-22== cookies from this recipe!
# [embedded html to pdf](https://www.encodian.com/blog/create-and-insert-a-document-approval-sheet-using-powerautomate-approval-data/)
|
- some text
| Column One | Column Two |
| --- | --- |
| data cell one | data cell two |
③ ④ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩
⓪
① ② ③ ⑤ ⑥ ⑦ ⑧ ⑨ ⑩
⑪ ⑫ ⑬ ⑭ ⑮ ⑯ ⑰ ⑱ ⑲
🄰 🄱 🄲 🄳 🄴 🄵 🄶 🄷 🄸 🄹 🄺 🄻 🄼 🄽 🄾 🄿 🅀 🅁 🅂 🅃 🅄 🅅 🅆 🅇 🅈 🅉
Ⓐ Ⓑ Ⓒ Ⓓ Ⓔ
⓿
❶ ❷ ❸ ❹ ❺ ❻ ❼ ❽ ❾ ❿
⓫ ⓬ ⓭ ⓮ ⓯ ⓰ ⓱ ⓲ ⓳ ⓴
Think of Pieces as a technical Tinkerbell from Peter Pan[^2].
[^2]: This is a fairytale about a forever young boy who takes a group of British children to his home island, Neverland.
Depending on how much cookie dough you eat, you’ll have about ==20-22== cookies from this recipe!
# [embedded html to pdf](https://www.encodian.com/blog/create-and-insert-a-document-approval-sheet-using-powerautomate-approval-data/)
b[^2]
🄱 outdoor|Pakai HDPE| Symbol| |:-----------|:-----------------|:-------|:---------| |Floating cage| Outdoor | ① | |Keramba dasar
0.5m from bottom|Outdoor | ② | |Keramba tanjab| |③ | |tambak/pond | Outdoor |④ | |hapa di tambak| Outdoor |⑤ | | | Indoor | ---------------- --------------- |Fish/Crustean | FCR wet/dry/
pellet(sink/float)|Culture Ponds
days/weeks/months|Stage Description
Larvae,Juvinele,Adult/
Temperature|Need fish meal| |:-------------|:------------------------------|:-----------|:-----------|:----------| |Tilapia |Easier and
no need much knowledge | |No need fish meal, soybeaan protein| |Tuna | 10:1|[swift memory safe](https://forums.swift.org/t/prospective-vision-optional-strict-memory-safety-for-swift/75090)|yes, due to fish oil| |Salmon|2:1|-|yes, due to fish oil| |**Red Grouper(bottom dweller)**| | |yes, due to fish oil, candidate for cages like lobster| |**Jack Red Snapper(Kakap merah bakau)
(Lutjanus argentimaculatus)**| ||Telur sifat apung seperti kakap putih
juvinelle bisa hidup di air tawar| |Black grouper (top dweller)| || | |ikan tirusan| | | | |Asian seabass male at birth vs black seabass female at birth||| -------------- [Grow-out Culture](https://repository.seafdec.org.ph/bitstream/handle/10862/36/adsea91p003-012.pdf?sequence=1&isAllowed=y) --- Polyculture of sea bass using tilapia as forage fish is often practiced. Growth and survival were higher among sea bass reared with tilapia in the same compartment than among those reared in ponds with net partition to separate the larger prey from predator (Triño, unpublished). [Tiger Prawn floating cage growout culture](https://eprints.cmfri.org.in/10937/2/46459-150550-1-PB%20%283%29.pdf) ## Markdown Heading * ~~strikthrough~~ * Lake Como [84km north of Milan] and Lake Garda [137km from Milan] * [style in flowchart mermaid instead of graph](https://www.freecodecamp.org/news/use-mermaid-javascript-library-to-create-flowcharts/) * [SAP Ui5 architecture](https://www.tutorialspoint.com/sap_ui5/sap_ui5_architecture.htm) * [SAp U5 hana](https://sapui5.hana.ondemand.com/sdk/#/topic/28b59ca857044a7890a22aec8cf1fee9) * [sap ui5 example](https://openui5.org/) * [sap flori tutorial](https://ui5.sap.com//#/topic/3da5f4be63264db99f2e5b04c5e853db) * [Flori web design](https://experience.sap.com/fiori-design-web/action-placement/) * [shell flori tool bar](https://experience.sap.com/fiori-design-web/flexible-column-layout/) * [sap grid view html](https://experience.sap.com/fiori-design-web/flexible-grid/) * [Dioxus](https://codesandbox.io/p/github/mattdanielbrown/Dioxus-Example-Projects/master?file=%2Fecommerce-site%2Fsrc%2Fmain.rs%3A19%2C26) * [Bookmark](https://www.croftsoft.com/library/tutorials/rust-dioxus-project-setup/) * [docsite dioxus tailwind components](https://github.com/42Angouleme/dioxus-tw-components)
 ## Sebass vs Snapper
- diffferent
- which one taste better
|The table below provides a quick comparison of the availability and cost of snapper and grouper:|
|------------------------------------------------------------------------------------------------|
|Fish| Availability| Cost|
|----|--------------|--------|
|Snapper| Common in Gulf of Mexico and Caribbean| Higher price due to demand and difficulty of catching|
|Grouper| Common in Atlantic and Pacific oceans| More affordable due to abundance and ease of catching|
## Snapper vs Grouper
Both of these species are known for having firm yet moist meat while having the added health benefit of being low in fat. Snapper is slightly sweeter and the meat more delicate, especially when grilled, than Grouper. Most compare its flavor and texture to be similar to that of halibut or sea bass. Grouper on the other hand is milder or more subtle in flavor making it perfect for absorbing either dressings or marinades
[Singapore Snapper Hatchery](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/science-poster_development-of-hatchery-technology-for-crs-v4-revised-29-june-2022-003.pdf)
[Spawing](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/application-of-endocrine-techniques-in-fish-spawning.pdf)
[Treatment](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/monogeneans-disease-card-v2-english.pdf)
[Start](https://www.sfa.gov.sg/docs/default-source/food-farming/industry-guides/sfa-sea-farming-guide326598c6-3d7b-4fb5-8f80-9b3228fd595c.pdf/)
## Safe C++
1. A new "safe" context where only a rigorously safe
subset of C++ is allowed
2. Borrow checking to prevent use-after-free bugs
3. Initialization analysis for type safety
4. Pattern matching, choice types, and borrowing
5. Thread-safety features similar to Rust's
send and sync type traits
6. Explicit mutation for references
7. A model for object relocation
## hardshell vs softshell (or just molting shrimp) result in high and low salinity shirmp culture
Hard shell lobsters also have less water weight, while soft
shell lobsters contain more water. This difference in yield is
reflected in the lower pricing of soft shell lobsters. For comparison, the average
yield for hard shells is roughly 20% to 28% meat, while soft shells contain around 15% to
20% meat. 5 Jun 2018
Low Salinity
Less then 5ppt:
1. Apply sea salt
* 100 kg/Acre weekly twice any time
2. Add high salinity water New worldlife
3. Feed supplement sea salt for low salinity
* 50g/kg feed
5.Fluctuation Water Parameters
6.Big Size PL Neworldlife
7.Insufficient Minerals
8.Use One or More then Mother Shrimps
Batam
---
* Industry sampai jembatan 5.
* fiber malaysia kapalnya lebih tebal daan berat jadi lebih stabil
* Mesin kapal bisa jadi dua atau 1 (40 HP atau (2x 15HP)
* Pulau Ketam malaysia ( hatchery and growth out sea pans) - outer island near Kuala Lumpur ( near Riau)
-[serial port in rust](https://users.rust-lang.org/t/how-to-use-serial-port-in-dioxus-desktop-app/99845/3)
# [ikan tirusan-(Otolithoides pama)](https://www.mongabay.co.id/2024/09/16/ikan-tirusan-harga-selangit-jadi-primadona-khawatir-penangkapan-berlebih/)
- [(Otolithoides pama)](https://www.themimu.info/sites/themimu.info/files/assessment_file_attachments/GOM_Pama_Croaker_croaker.pdf)
- [xxx](https://eprints.cmfri.org.in/16358/1/MFIS%20252_2022_Subal%20Kumar%20Roul.pdf)
- gelambung laki jauh lebih mahaldari yang betina
Para nelayan di Riau, seperti Indragiri Hilir, belakangan ini ‘berburu’ mendapatkan ikan tirusan atau tirus karena harga yang selangit. Ikan dengan nama latin Otolithoides pama, termasuk famili sciaenidae, harga per ekor bisa mencapai puluhan juta rupiah.
Yang bernilai tinggi dari ikan tirusan adalah gelembung (limpanya). Pembeli atau pengepul biasa mengupah orang yang ahli lagi untuk membelah perut ikan ini.
Hasil pencatatan BPPMHKP Pekanbaru, pengiriman gelembung ikan sepanjang 2019-2023 mencapai 1.204 kali atau 36.895 kilogram. Sumbernya dari enam daerah penghasil ikan di
1. Tembilahan,
2. Bengkalis,
3. Dumai,
4. Selat Panjang,
5. Pekanbaru dan
6. Panipahan.
Setidaknya ada enam pengirim utama mendominasi dalam perdagangan gelembung ikan di Riau.
Data ini tidak spesifik menyebut jenis ikan penghasil gelembung renang itu. Selain tirusan, gelembung yang biasa diperdagangkan juga produk turunan dari ikan malong (Muraenesox cinereus).
# [Ikan Malong (Muraenesox cinereus)](https://indiabiodiversity.org/species/show/232787)
Malong ( Muraenesox cinereus )adalah spesies ikan Sidat dalam keluarga pike conger, Muraenesocidae .
Mereka terutama hidup di dasar lunak di perairan laut dan air payau hingga kedalaman 800 m (2.600 ft), tapi bisa masuk ke air tawar.
# [Gamat Hitam- Teripang hitam ](https://www.mitrausahatani.com/teripang-berhasil-dibudidayakan/)
- [teripang](https://youtu.be/3O4dGg5DJc4?si=gpO6H6sVmOFNFKmP)q
## Sebass vs Snapper
- diffferent
- which one taste better
|The table below provides a quick comparison of the availability and cost of snapper and grouper:|
|------------------------------------------------------------------------------------------------|
|Fish| Availability| Cost|
|----|--------------|--------|
|Snapper| Common in Gulf of Mexico and Caribbean| Higher price due to demand and difficulty of catching|
|Grouper| Common in Atlantic and Pacific oceans| More affordable due to abundance and ease of catching|
## Snapper vs Grouper
Both of these species are known for having firm yet moist meat while having the added health benefit of being low in fat. Snapper is slightly sweeter and the meat more delicate, especially when grilled, than Grouper. Most compare its flavor and texture to be similar to that of halibut or sea bass. Grouper on the other hand is milder or more subtle in flavor making it perfect for absorbing either dressings or marinades
[Singapore Snapper Hatchery](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/science-poster_development-of-hatchery-technology-for-crs-v4-revised-29-june-2022-003.pdf)
[Spawing](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/application-of-endocrine-techniques-in-fish-spawning.pdf)
[Treatment](https://www.sfa.gov.sg/docs/default-source/food-science-and-technology/monogeneans-disease-card-v2-english.pdf)
[Start](https://www.sfa.gov.sg/docs/default-source/food-farming/industry-guides/sfa-sea-farming-guide326598c6-3d7b-4fb5-8f80-9b3228fd595c.pdf/)
## Safe C++
1. A new "safe" context where only a rigorously safe
subset of C++ is allowed
2. Borrow checking to prevent use-after-free bugs
3. Initialization analysis for type safety
4. Pattern matching, choice types, and borrowing
5. Thread-safety features similar to Rust's
send and sync type traits
6. Explicit mutation for references
7. A model for object relocation
## hardshell vs softshell (or just molting shrimp) result in high and low salinity shirmp culture
Hard shell lobsters also have less water weight, while soft
shell lobsters contain more water. This difference in yield is
reflected in the lower pricing of soft shell lobsters. For comparison, the average
yield for hard shells is roughly 20% to 28% meat, while soft shells contain around 15% to
20% meat. 5 Jun 2018
Low Salinity
Less then 5ppt:
1. Apply sea salt
* 100 kg/Acre weekly twice any time
2. Add high salinity water New worldlife
3. Feed supplement sea salt for low salinity
* 50g/kg feed
5.Fluctuation Water Parameters
6.Big Size PL Neworldlife
7.Insufficient Minerals
8.Use One or More then Mother Shrimps
Batam
---
* Industry sampai jembatan 5.
* fiber malaysia kapalnya lebih tebal daan berat jadi lebih stabil
* Mesin kapal bisa jadi dua atau 1 (40 HP atau (2x 15HP)
* Pulau Ketam malaysia ( hatchery and growth out sea pans) - outer island near Kuala Lumpur ( near Riau)
-[serial port in rust](https://users.rust-lang.org/t/how-to-use-serial-port-in-dioxus-desktop-app/99845/3)
# [ikan tirusan-(Otolithoides pama)](https://www.mongabay.co.id/2024/09/16/ikan-tirusan-harga-selangit-jadi-primadona-khawatir-penangkapan-berlebih/)
- [(Otolithoides pama)](https://www.themimu.info/sites/themimu.info/files/assessment_file_attachments/GOM_Pama_Croaker_croaker.pdf)
- [xxx](https://eprints.cmfri.org.in/16358/1/MFIS%20252_2022_Subal%20Kumar%20Roul.pdf)
- gelambung laki jauh lebih mahaldari yang betina
Para nelayan di Riau, seperti Indragiri Hilir, belakangan ini ‘berburu’ mendapatkan ikan tirusan atau tirus karena harga yang selangit. Ikan dengan nama latin Otolithoides pama, termasuk famili sciaenidae, harga per ekor bisa mencapai puluhan juta rupiah.
Yang bernilai tinggi dari ikan tirusan adalah gelembung (limpanya). Pembeli atau pengepul biasa mengupah orang yang ahli lagi untuk membelah perut ikan ini.
Hasil pencatatan BPPMHKP Pekanbaru, pengiriman gelembung ikan sepanjang 2019-2023 mencapai 1.204 kali atau 36.895 kilogram. Sumbernya dari enam daerah penghasil ikan di
1. Tembilahan,
2. Bengkalis,
3. Dumai,
4. Selat Panjang,
5. Pekanbaru dan
6. Panipahan.
Setidaknya ada enam pengirim utama mendominasi dalam perdagangan gelembung ikan di Riau.
Data ini tidak spesifik menyebut jenis ikan penghasil gelembung renang itu. Selain tirusan, gelembung yang biasa diperdagangkan juga produk turunan dari ikan malong (Muraenesox cinereus).
# [Ikan Malong (Muraenesox cinereus)](https://indiabiodiversity.org/species/show/232787)
Malong ( Muraenesox cinereus )adalah spesies ikan Sidat dalam keluarga pike conger, Muraenesocidae .
Mereka terutama hidup di dasar lunak di perairan laut dan air payau hingga kedalaman 800 m (2.600 ft), tapi bisa masuk ke air tawar.
# [Gamat Hitam- Teripang hitam ](https://www.mitrausahatani.com/teripang-berhasil-dibudidayakan/)
- [teripang](https://youtu.be/3O4dGg5DJc4?si=gpO6H6sVmOFNFKmP)q
 - [Teripang Hatchery video](https://www.youtube.com/watch?feature=shared&v=iG1IX73R1D4)
- [Seacuucumber Processing](https://youtu.be/hkuZCiMJy1s?si=U7Xo-IzJ5vAngoRn)
- [Seacucumber investment](https://www.youtube.com/watch?v=u5MmzsAoXOk)
[seacucumber](https://www.ctsa.org/files/publications/SeaCucumberHatcheryManual.pdf)
- [start business seacucumber](https://youtu.be/_Qa1R2EEBCg?si=hB4PWzQhXBMunqJA)
- [Brackish shrimp culture](http://www.agritech.tnau.ac.in/fishery/fish_cul_brackish_shrimps_stock.html)
Market trends
---
The dried air bladders called fish maws are exported mainly to
* China and Singapore for the manufacture of isinglass, which is used in beverage and cosmetic
industry as well as Chinese traditional medicines (Dutta et al., 2014). The lesser and medium sciaenids are mostly
sold in fresh condition at the local markets or iced and transported to distant interior markets of Odisha, West
- [x] Write the press release
- [ ] Update the website
- [ ] Contact the media
# [Tilapia](https://youtu.be/7SOVLYYgyCE?si=Z3zplqT-GU7pJFfF)
# [Catfish](https://www.youtube.com/watch?v=YoI2QocTmlQ)
# [sandfish](https://www.sciencedirect.com/science/article/pii/S2352513424000334)
Grow-out operations in central Vietnam are capable of producing market-size sandfish (350–400 g), from 2–5 g juveniles, after approximately 12 months in earthen pond-based culture.
However, the onset of the wet season in Vietnam (typically from May to early November) and resulting reduction in the salinity of culture water,
may impact productivity through reduced growth rates and survival (Mills et al., 2012).
Vietnamese farmers have consequently developed an ‘advanced nursery’ phase, where 2–5 g juveniles are cultured at high density in ponds with organic matter-rich sediment (>50,000 ind./ha compared
to ∼10,000 ind./ha which is the standard grow-out density), until they reach a size of ca. 50 g.
This permits ‘short-cropping’ during the grow-out phase, because when larger juveniles are grown out, they can reach the target harvest weight of 350–400 g within 7–9 months
and experience higher rates of survival (Mills et al., 2012).
The goal of this study was therefore to undertake a preliminary assessment of the feasibility of supplemental feeding to support short-cropping culture of sandfish in Vietnam by:
1. evaluating the growth and survival performance of sandfish juveniles offered two different types of locally-sourced seaweed-based supplemental feeds (Sargassum sp. and Gracilaria verrucosa)
processed by both fermentation and pulverisation;
2. determining an optimal stocking density for juveniles offered supplemental feeding; and
3. applying the best-performing supplemental feed, determined from (1) during large-scale earthen-pond culture to assess the feasibility of commercial-scale short cropping.
Supplemental feed preparation
===
Supplemental feeds were prepared from commercially available wild-sourced seaweed collected from coastal regions in Khánh Hòa Province, Vietnam, which is readily available in dried form (Hong and Ha, 2022). Fresh seaweed is first cleaned at the collection site using seawater to remove sand, debris, epiphytic growths and other extraneous matter attached to thalli and, prior to sun-drying, was rinsed with freshwater to remove salt residue. Both species of dried seaweed were powdered to a particle size of 350–450 µm using a high-speed dry grinding machine (2 kg capacity; 4000 W; model RRH2000A; Riri Hong Ltd) to produce the pulverised supplemental feeds. The fermented diets were prepared in batches as required. To prepare a single batch of fermented feed, 10 kg of dried seaweed powder was mixed with 2.5 g of a commercially-available livestock feed probiotic mixture (BIO-MEN animal feed brewing yeast/Men ủ thức ăn chăn nuôi BIO-MEN manufactured by Vườn Sinh Thái/Eco Garden, Vietnam), and 10 L of freshwater. The probiotic mixture comprised Saccharomyces boulardii (at a concentration of 1010-1014 Colony Forming Units; CFU), Lactobacillus acidophilus (1010 CFU) and Bacillus subtilis (1010 CFU) per g, respectively. This product had a maximum moisture content of 10%, sand/grit content of 2% and used corn flour and rice bran as the probiotic carrier agents (https://vuonsinhthai.com.vn/men-u-vi-sinh-thao-duoc-u-chin-thuc-an-chan-nuoi.html
).
# Pearl Oyster
# [Scallop](https://www.e-fas.org/archive/view_article?pid=fas-27-11-709)
1. Chlamys farreri,
2. Argopecten irradians,
3. Pactinopecten yessoensis
4. and Chlamys nobilis
# use of oyster shell
The easiest way to avoid this problem is to only use aged shell in your setting tanks. Every shell used in the system should be aged
at least a full year out in the open where it will be is exposed to weather and other factors that will remove the organic material from the shells. Once this ageing process
is completed, it is best if the shells are washed or cleaned to remove as much dirt and small shell fragments from the cultch as possible. When you have clean cultch material
it is ready to be containerized for use in your setting system.
## Amorphous calcium carbonate (ACC)
is a non-crystalline form of calcium carbonate, and it is composed of aggregated nano-size primary particles.
Here, we evaluated its anti-cancer effect postulated relative to its buffering capabilities in lung cancer.
# Aeration
The most common approach is to use aeration. Most setting tanks are fairly shallow (6 feet deep or less) and this lends itself to aeration by rotary blower. Rotary blowers are used
because they provide high volumes of air at low pressure. They are more efficient than compressors and do not introduce oil into the system.
Blowers should be sized to provide a gentle rolling movement throughout the entire setting tank. When installing aeration, make sure that water from the setting tanks is
not allowed by gravity to flow backwards into the blower when the blower is off.
The easiest ways to do this are either to install the blower above the highest water level of the setting tanks or to add a section of pipe that extends above the high water mark.
Getting air from the blower to the tanks is usually done through PVC pipes. Pipes
should be sized according to the volume of the setting tanks but, with smaller tanks
capable of holding 200-300 shell bags, 1.5 to 2 inch inside diameter pipes are
sufficient. Larger tanks will require larger diameter aeration lines. Blowers used in
remote setting do not create much pressure and therefore the use of larger pipe is
required. Pipes should be drilled with 3/8” holes about every 8 to 12 inches along
the length of the pipe to provide adequate aeration. Aeration pipes should be spaced
apart to allow a gentle flow to be created when the air is on. For example, a 12 foot
diameter tank with 3 or 4 pipes spaced at the bottom of the tank provides sufficient
aeration for good settlement to occur.
It is important that aeration pipes used inside the tanks NOT be glued together. These pipes will fill with sediment, have oyster larvae settle on them, and other things that
require them to be cleaned inside and out. In fact, each time a setting tank is broken down and reloaded with cultch, the air pipes should
be taken apart and thoroughly cleaned inside and out. This can be done with a brush on a
rope and fresh water, another good reason to use larger diameter piping. Care should be taken to remove ALL newly settled oyster spat
from the pipes and any other surface in the setting tank. If the tanks are re-used these spat will continue to grow. These spat
are voracious feeders and will compete with the next batch of larval oysters for the food in the tank and as they get bigger will provide substrate for oysters to settle on
# Instant algae
Instant algae is your easy solution for replacing or supplementing live microalgae in commercial fish, shrimp, shellfish and ornamental hatcheries, universities and research centers. It is real marine microalgae, grown under laboratory conditions and concentrated for easy storage and long life. Instant algae is not alive and cannot be used as starter cultures – it is an inexpensive bulk feed.
Nannochloropsis
These are small green algaes that are extensively used in the aquaculture industry for growing small zooplankton such as rotifers and for greenwater. They are also used in reef tanks for feeding corals and other filter feeders. It is 3600 times as dense as cultured algae, so 1 liter replaces almost 4 metric tons of live algae! Available in 1-quart & 1-pint bottles, and 1-liter bags. Nannochloropsis is the single best food for growing rotifers. It has a very high EPA level and can be frozen for long term storage. It also works well for greenwater.
Shellfish hatcheries: works very well with mussels. It has not been traditionally used with oysters and clams, however a study currently being done at a university in Los Angeles indicates that it can work as well or better than Isochrysis and Pavlova with larvae and post set. Storage: instant algae nannochloropsis can be stored in a refrigerator for 3 months (best if stored at -1° to 3°C) or it can be frozen for longer shelf life (2+ years). When frozen, the algae will “hard-freeze” like ice.
Pavlova
Pavlova is a small golden/brown flagellate that is very similar to Isochrysis. It has a very high DHA profile and is excellent for enriching rotifers and other zooplankton. It is very difficult to develop the Pavlova, that is the reason why it is not produced by many incubation facilities. Pavlova is similar in nutritional profile to Isochrysis, however it has a more sophisticated sterol composition so it is very popular with cold water fish hatcheries (such as cod) for enriching rotifers.
Pavlova has both a high DHA and EPA profile so its good for enriching zooplankton. We have received very good reports on larval survival from hatcheries that use it to enrich brine shrimp. Pavlova works very well for oysters, clams, mussels and scallops. It is also used to increase the DHA/EPA levels in broodstock.
Isochrysis
Isochrysis is a small golden/brown flagellate that is very commonly used in the aquaculture industry. It is high in DHA and often used to enrich zooplankton such as rotifers or Artemia. Used for DHA enrichment of rotifers.
It is also a good size for feeding brine shrimp and copepods. Isochrysis is the most commonly used algae for oysters, clams, mussels and scallops.
Tetraselmis
Tetraselmis is a large green flagellate with a very high lipid level. It also contains natural amino acids that stimulate feeding in marine animals. It is an excellent feed for larval shrimp. Used in conjunction with Nannochloropsis for producing rotifers.
It is also a good size for feeding brine shrimp. A standard feed for oysters, clams, mussels and scallops.
Rotifer Diet HD
Rotifer Diet™ HD is a marine microalgae feed product for producing high quality, high density, low cost rotifers. Rotifer Diet HD is a combination of the microalgae species Nannochloropsis & Tetraselmis and results in a 20% increase in rotifer volume over pure Nannochloropsis.
It is a 100% complete feeding solution, needing no other feed additives to produce dense cultures. Rotifer Diet HD was specifically designed for high density rotifer systems, but also works exceptionally well in low density and batch cultures.
Thalassiosira weissflogii
Thalassiosira weissflogii is a large diatom (6-20μm x 8-15μm) that is used in the shrimp and shellfish larviculture industry. This algae is considered by several hatcheries to be the single best algae for larval shrimp. The large cell size (16 times the biomass of Chaetoceros and 3 times the biomass of Tetraselmis) extends the algae feeding period until the end of the PL stage. During the winter, this algae is about 15 microns, but shrinks to about 5 microns during the summer.
The color of TW varies from brown to green to yellow, depending on the amount of chlorophyll in the culture. This color change does not in any way affect the quality of the algae. Good for feeding copepods and brine shrimp. Due to its large size, TW is a very good feed for post-set (200 microns and larger) oysters, clams, mussels, scallops, and for broodstock conditioning.
Shellfish Diet
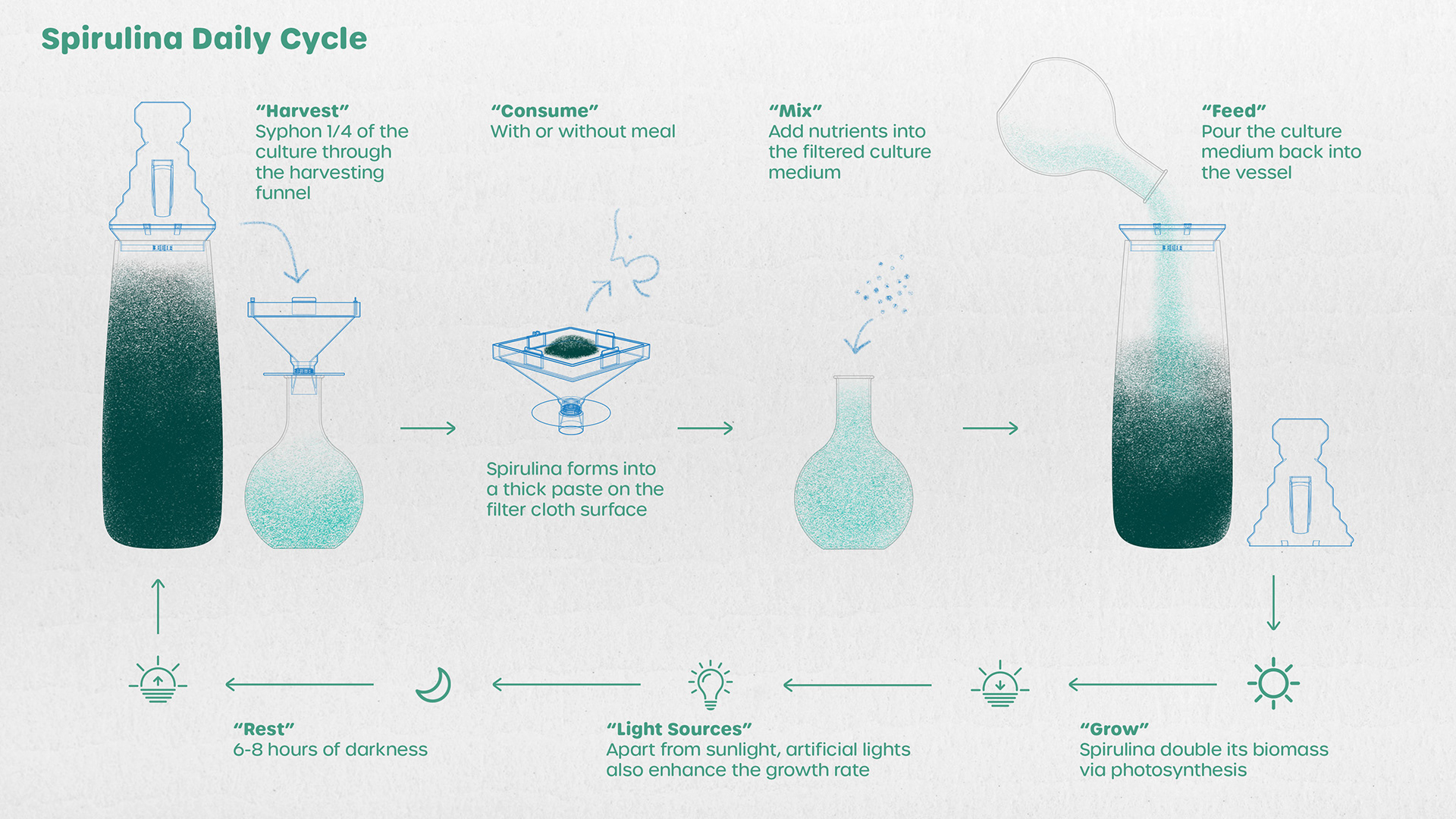
Shellfish diet is a mix of five marine microalgae that all have demonstrated success with a variety of shellfish including oysters, clams, mussels and scallops. A mixed diet provides a much better nutritional profile for all types of shellfish, increasing both growth rates and survival. Shellfish diet can be used with preset larvae all the way up through broodstock and will typically perform as well as algae so it can be used as a complete live algae replacement. 1/4 of Shellfish diet will replace the equivalent to 1800 liters of dense algae culture. This product is available in plastic bottles of 1/4, 1-pint and 1-gallon. The quantity of cells changes depending on the time of the year, but is approximately 2 billions of cells per ml.
# Sargassum Powder
- [Teripang Hatchery video](https://www.youtube.com/watch?feature=shared&v=iG1IX73R1D4)
- [Seacuucumber Processing](https://youtu.be/hkuZCiMJy1s?si=U7Xo-IzJ5vAngoRn)
- [Seacucumber investment](https://www.youtube.com/watch?v=u5MmzsAoXOk)
[seacucumber](https://www.ctsa.org/files/publications/SeaCucumberHatcheryManual.pdf)
- [start business seacucumber](https://youtu.be/_Qa1R2EEBCg?si=hB4PWzQhXBMunqJA)
- [Brackish shrimp culture](http://www.agritech.tnau.ac.in/fishery/fish_cul_brackish_shrimps_stock.html)
Market trends
---
The dried air bladders called fish maws are exported mainly to
* China and Singapore for the manufacture of isinglass, which is used in beverage and cosmetic
industry as well as Chinese traditional medicines (Dutta et al., 2014). The lesser and medium sciaenids are mostly
sold in fresh condition at the local markets or iced and transported to distant interior markets of Odisha, West
- [x] Write the press release
- [ ] Update the website
- [ ] Contact the media
# [Tilapia](https://youtu.be/7SOVLYYgyCE?si=Z3zplqT-GU7pJFfF)
# [Catfish](https://www.youtube.com/watch?v=YoI2QocTmlQ)
# [sandfish](https://www.sciencedirect.com/science/article/pii/S2352513424000334)
Grow-out operations in central Vietnam are capable of producing market-size sandfish (350–400 g), from 2–5 g juveniles, after approximately 12 months in earthen pond-based culture.
However, the onset of the wet season in Vietnam (typically from May to early November) and resulting reduction in the salinity of culture water,
may impact productivity through reduced growth rates and survival (Mills et al., 2012).
Vietnamese farmers have consequently developed an ‘advanced nursery’ phase, where 2–5 g juveniles are cultured at high density in ponds with organic matter-rich sediment (>50,000 ind./ha compared
to ∼10,000 ind./ha which is the standard grow-out density), until they reach a size of ca. 50 g.
This permits ‘short-cropping’ during the grow-out phase, because when larger juveniles are grown out, they can reach the target harvest weight of 350–400 g within 7–9 months
and experience higher rates of survival (Mills et al., 2012).
The goal of this study was therefore to undertake a preliminary assessment of the feasibility of supplemental feeding to support short-cropping culture of sandfish in Vietnam by:
1. evaluating the growth and survival performance of sandfish juveniles offered two different types of locally-sourced seaweed-based supplemental feeds (Sargassum sp. and Gracilaria verrucosa)
processed by both fermentation and pulverisation;
2. determining an optimal stocking density for juveniles offered supplemental feeding; and
3. applying the best-performing supplemental feed, determined from (1) during large-scale earthen-pond culture to assess the feasibility of commercial-scale short cropping.
Supplemental feed preparation
===
Supplemental feeds were prepared from commercially available wild-sourced seaweed collected from coastal regions in Khánh Hòa Province, Vietnam, which is readily available in dried form (Hong and Ha, 2022). Fresh seaweed is first cleaned at the collection site using seawater to remove sand, debris, epiphytic growths and other extraneous matter attached to thalli and, prior to sun-drying, was rinsed with freshwater to remove salt residue. Both species of dried seaweed were powdered to a particle size of 350–450 µm using a high-speed dry grinding machine (2 kg capacity; 4000 W; model RRH2000A; Riri Hong Ltd) to produce the pulverised supplemental feeds. The fermented diets were prepared in batches as required. To prepare a single batch of fermented feed, 10 kg of dried seaweed powder was mixed with 2.5 g of a commercially-available livestock feed probiotic mixture (BIO-MEN animal feed brewing yeast/Men ủ thức ăn chăn nuôi BIO-MEN manufactured by Vườn Sinh Thái/Eco Garden, Vietnam), and 10 L of freshwater. The probiotic mixture comprised Saccharomyces boulardii (at a concentration of 1010-1014 Colony Forming Units; CFU), Lactobacillus acidophilus (1010 CFU) and Bacillus subtilis (1010 CFU) per g, respectively. This product had a maximum moisture content of 10%, sand/grit content of 2% and used corn flour and rice bran as the probiotic carrier agents (https://vuonsinhthai.com.vn/men-u-vi-sinh-thao-duoc-u-chin-thuc-an-chan-nuoi.html
).
# Pearl Oyster
# [Scallop](https://www.e-fas.org/archive/view_article?pid=fas-27-11-709)
1. Chlamys farreri,
2. Argopecten irradians,
3. Pactinopecten yessoensis
4. and Chlamys nobilis
# use of oyster shell
The easiest way to avoid this problem is to only use aged shell in your setting tanks. Every shell used in the system should be aged
at least a full year out in the open where it will be is exposed to weather and other factors that will remove the organic material from the shells. Once this ageing process
is completed, it is best if the shells are washed or cleaned to remove as much dirt and small shell fragments from the cultch as possible. When you have clean cultch material
it is ready to be containerized for use in your setting system.
## Amorphous calcium carbonate (ACC)
is a non-crystalline form of calcium carbonate, and it is composed of aggregated nano-size primary particles.
Here, we evaluated its anti-cancer effect postulated relative to its buffering capabilities in lung cancer.
# Aeration
The most common approach is to use aeration. Most setting tanks are fairly shallow (6 feet deep or less) and this lends itself to aeration by rotary blower. Rotary blowers are used
because they provide high volumes of air at low pressure. They are more efficient than compressors and do not introduce oil into the system.
Blowers should be sized to provide a gentle rolling movement throughout the entire setting tank. When installing aeration, make sure that water from the setting tanks is
not allowed by gravity to flow backwards into the blower when the blower is off.
The easiest ways to do this are either to install the blower above the highest water level of the setting tanks or to add a section of pipe that extends above the high water mark.
Getting air from the blower to the tanks is usually done through PVC pipes. Pipes
should be sized according to the volume of the setting tanks but, with smaller tanks
capable of holding 200-300 shell bags, 1.5 to 2 inch inside diameter pipes are
sufficient. Larger tanks will require larger diameter aeration lines. Blowers used in
remote setting do not create much pressure and therefore the use of larger pipe is
required. Pipes should be drilled with 3/8” holes about every 8 to 12 inches along
the length of the pipe to provide adequate aeration. Aeration pipes should be spaced
apart to allow a gentle flow to be created when the air is on. For example, a 12 foot
diameter tank with 3 or 4 pipes spaced at the bottom of the tank provides sufficient
aeration for good settlement to occur.
It is important that aeration pipes used inside the tanks NOT be glued together. These pipes will fill with sediment, have oyster larvae settle on them, and other things that
require them to be cleaned inside and out. In fact, each time a setting tank is broken down and reloaded with cultch, the air pipes should
be taken apart and thoroughly cleaned inside and out. This can be done with a brush on a
rope and fresh water, another good reason to use larger diameter piping. Care should be taken to remove ALL newly settled oyster spat
from the pipes and any other surface in the setting tank. If the tanks are re-used these spat will continue to grow. These spat
are voracious feeders and will compete with the next batch of larval oysters for the food in the tank and as they get bigger will provide substrate for oysters to settle on
# Instant algae
Instant algae is your easy solution for replacing or supplementing live microalgae in commercial fish, shrimp, shellfish and ornamental hatcheries, universities and research centers. It is real marine microalgae, grown under laboratory conditions and concentrated for easy storage and long life. Instant algae is not alive and cannot be used as starter cultures – it is an inexpensive bulk feed.
Nannochloropsis
These are small green algaes that are extensively used in the aquaculture industry for growing small zooplankton such as rotifers and for greenwater. They are also used in reef tanks for feeding corals and other filter feeders. It is 3600 times as dense as cultured algae, so 1 liter replaces almost 4 metric tons of live algae! Available in 1-quart & 1-pint bottles, and 1-liter bags. Nannochloropsis is the single best food for growing rotifers. It has a very high EPA level and can be frozen for long term storage. It also works well for greenwater.
Shellfish hatcheries: works very well with mussels. It has not been traditionally used with oysters and clams, however a study currently being done at a university in Los Angeles indicates that it can work as well or better than Isochrysis and Pavlova with larvae and post set. Storage: instant algae nannochloropsis can be stored in a refrigerator for 3 months (best if stored at -1° to 3°C) or it can be frozen for longer shelf life (2+ years). When frozen, the algae will “hard-freeze” like ice.
Pavlova
Pavlova is a small golden/brown flagellate that is very similar to Isochrysis. It has a very high DHA profile and is excellent for enriching rotifers and other zooplankton. It is very difficult to develop the Pavlova, that is the reason why it is not produced by many incubation facilities. Pavlova is similar in nutritional profile to Isochrysis, however it has a more sophisticated sterol composition so it is very popular with cold water fish hatcheries (such as cod) for enriching rotifers.
Pavlova has both a high DHA and EPA profile so its good for enriching zooplankton. We have received very good reports on larval survival from hatcheries that use it to enrich brine shrimp. Pavlova works very well for oysters, clams, mussels and scallops. It is also used to increase the DHA/EPA levels in broodstock.
Isochrysis
Isochrysis is a small golden/brown flagellate that is very commonly used in the aquaculture industry. It is high in DHA and often used to enrich zooplankton such as rotifers or Artemia. Used for DHA enrichment of rotifers.
It is also a good size for feeding brine shrimp and copepods. Isochrysis is the most commonly used algae for oysters, clams, mussels and scallops.
Tetraselmis
Tetraselmis is a large green flagellate with a very high lipid level. It also contains natural amino acids that stimulate feeding in marine animals. It is an excellent feed for larval shrimp. Used in conjunction with Nannochloropsis for producing rotifers.
It is also a good size for feeding brine shrimp. A standard feed for oysters, clams, mussels and scallops.
Rotifer Diet HD
Rotifer Diet™ HD is a marine microalgae feed product for producing high quality, high density, low cost rotifers. Rotifer Diet HD is a combination of the microalgae species Nannochloropsis & Tetraselmis and results in a 20% increase in rotifer volume over pure Nannochloropsis.
It is a 100% complete feeding solution, needing no other feed additives to produce dense cultures. Rotifer Diet HD was specifically designed for high density rotifer systems, but also works exceptionally well in low density and batch cultures.
Thalassiosira weissflogii
Thalassiosira weissflogii is a large diatom (6-20μm x 8-15μm) that is used in the shrimp and shellfish larviculture industry. This algae is considered by several hatcheries to be the single best algae for larval shrimp. The large cell size (16 times the biomass of Chaetoceros and 3 times the biomass of Tetraselmis) extends the algae feeding period until the end of the PL stage. During the winter, this algae is about 15 microns, but shrinks to about 5 microns during the summer.
The color of TW varies from brown to green to yellow, depending on the amount of chlorophyll in the culture. This color change does not in any way affect the quality of the algae. Good for feeding copepods and brine shrimp. Due to its large size, TW is a very good feed for post-set (200 microns and larger) oysters, clams, mussels, scallops, and for broodstock conditioning.
Shellfish Diet
Shellfish diet is a mix of five marine microalgae that all have demonstrated success with a variety of shellfish including oysters, clams, mussels and scallops. A mixed diet provides a much better nutritional profile for all types of shellfish, increasing both growth rates and survival. Shellfish diet can be used with preset larvae all the way up through broodstock and will typically perform as well as algae so it can be used as a complete live algae replacement. 1/4 of Shellfish diet will replace the equivalent to 1800 liters of dense algae culture. This product is available in plastic bottles of 1/4, 1-pint and 1-gallon. The quantity of cells changes depending on the time of the year, but is approximately 2 billions of cells per ml.
# Sargassum Powder
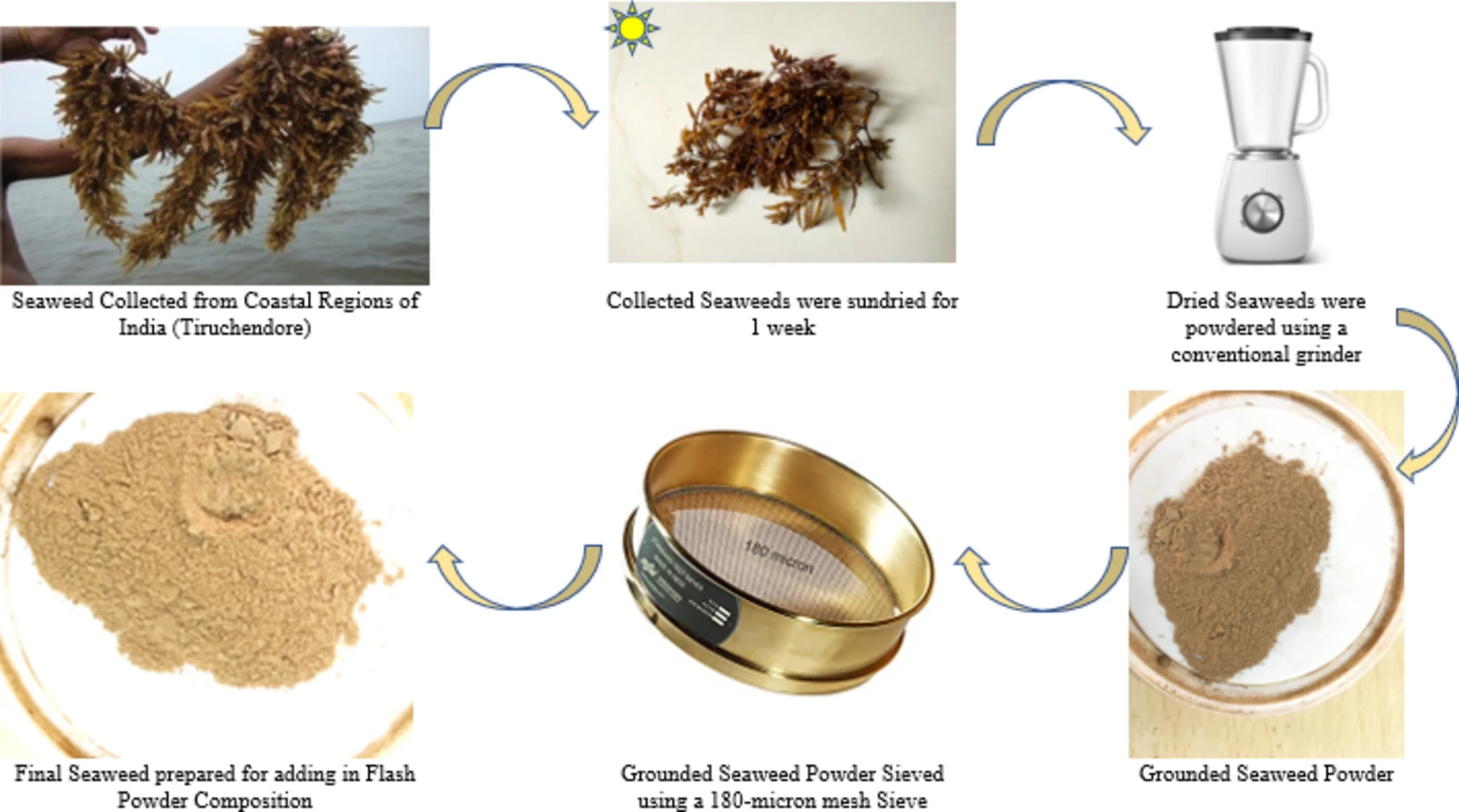 # Spirulina
# Spirulina
 Spirulina powder, a type of blue-green algae, is often cultivated in both freshwater and seawater environments. Seawater can be a viable and even advantageous medium for growing spirulina, particularly for reducing the need for additional nutrients. However, spirulina grown in seawater may have a higher iodine content and require careful drying processes to maintain qualit.
Seawater can be a cost-effective and potentially more sustainable medium for spirulina cultivation, especially when supplemented with nutrients. Studies have shown that spirulina can thrive in seawater, and in some cases, even exhibit increased carbohydrate production and biomass concentration compared to freshwater cultures.
The quality of spirulina, regardless of the cultivation medium, depends on factors like the source of the water, the cultivation methods, and the drying process. Spirulina harvested from clean sources and properly dried is essential for ensuring a high-quality product.
A large-scale cultivation experiment demonstrated that Spirulina platensis, strain SCS, can be adapted togrow in seawater enriched with a commercial compound fertilizer (N: Ρ: Κ = 12:12:12), NaHCO3 andFeSO4. To avoid excess precipitation, a compound fertilizer, which contained phosphate, was supplied bydiffusion through a tubular dialysis membrane and a small amount of NaHCO3 was added. The average yieldof biomass in 84 days of consecutive production was 10.3 g (dry weight) m~2 day"1. The protein and aminoacids contents in the product were 66.6% and 62.2% respectively. We have shown that outdoor cultivationof Spirulina in seawater has potential for industrial production without involving valuable farm land, employingless expensive culture medium which can be recycled and giving higher yield and higher quality products
Biochemical Changes
One of the most interesting side effects of culturing spirulina in seawater is that the carbohydrate content of it skyrockets while the protein content drops significantly.
[Spriluna](https://grow-organic-spirulina.com/product-category/live-spirulina-culture/)
Spirulina harvesting
---
involves separating the algae from its liquid growth medium. This is typically done through filtration or centrifugation, followed by drying to produce a powder or flakes. The harvested spirulina can then be used as a food supplement or in other application
[Harvest](https://spirulinasociety.org/how-to-harvest)
Spirulina powder, a type of blue-green algae, is often cultivated in both freshwater and seawater environments. Seawater can be a viable and even advantageous medium for growing spirulina, particularly for reducing the need for additional nutrients. However, spirulina grown in seawater may have a higher iodine content and require careful drying processes to maintain qualit.
Seawater can be a cost-effective and potentially more sustainable medium for spirulina cultivation, especially when supplemented with nutrients. Studies have shown that spirulina can thrive in seawater, and in some cases, even exhibit increased carbohydrate production and biomass concentration compared to freshwater cultures.
The quality of spirulina, regardless of the cultivation medium, depends on factors like the source of the water, the cultivation methods, and the drying process. Spirulina harvested from clean sources and properly dried is essential for ensuring a high-quality product.
A large-scale cultivation experiment demonstrated that Spirulina platensis, strain SCS, can be adapted togrow in seawater enriched with a commercial compound fertilizer (N: Ρ: Κ = 12:12:12), NaHCO3 andFeSO4. To avoid excess precipitation, a compound fertilizer, which contained phosphate, was supplied bydiffusion through a tubular dialysis membrane and a small amount of NaHCO3 was added. The average yieldof biomass in 84 days of consecutive production was 10.3 g (dry weight) m~2 day"1. The protein and aminoacids contents in the product were 66.6% and 62.2% respectively. We have shown that outdoor cultivationof Spirulina in seawater has potential for industrial production without involving valuable farm land, employingless expensive culture medium which can be recycled and giving higher yield and higher quality products
Biochemical Changes
One of the most interesting side effects of culturing spirulina in seawater is that the carbohydrate content of it skyrockets while the protein content drops significantly.
[Spriluna](https://grow-organic-spirulina.com/product-category/live-spirulina-culture/)
Spirulina harvesting
---
involves separating the algae from its liquid growth medium. This is typically done through filtration or centrifugation, followed by drying to produce a powder or flakes. The harvested spirulina can then be used as a food supplement or in other application
[Harvest](https://spirulinasociety.org/how-to-harvest)